Using soap to remove micropollutants from water
In spite of their low concentrations (about 0.01–100 µg/litre), micropollutants can be hazardous to the ecosystem and to human health. They come from a variety of sources like plastics during industrial manufacturing, pesticides, dyes, petrochemicals, and some heavy metals like Lead and Arsenic, potentially causing cancer, organ damage, or other adverse effects.
Currently, the most common practice for removing micropollutants from water is activated Carbon adsorption. Carbon filter, removing only 30% of micropollutants.
Activated Carbon requires high temperatures to produce and regenerate, requiring specialized equipment and consuming large amounts of energy. Reverse osmosis can also be used to remove micropollutants from water; however, "it doesn't lead to good elimination of this class of molecules, because of both their concentration and their molecular structure.
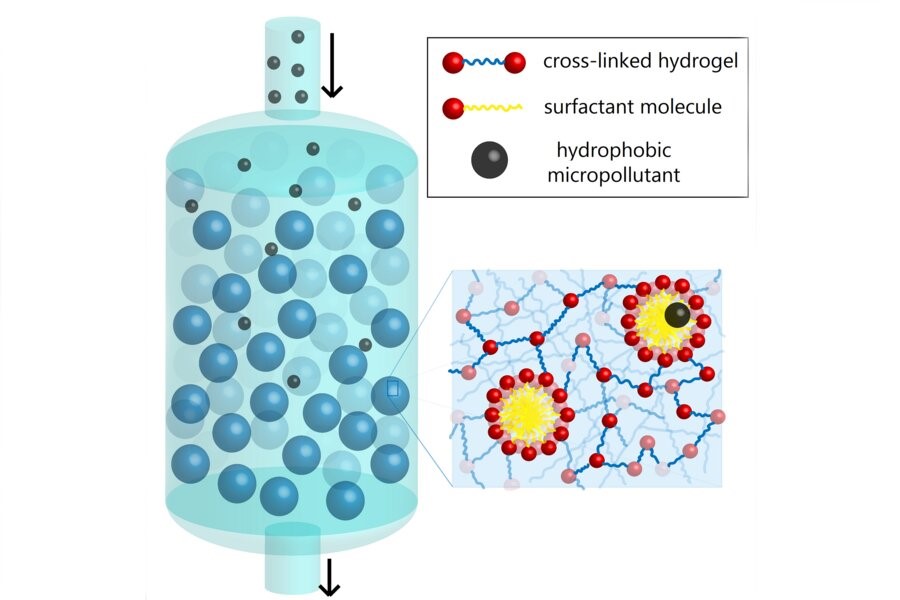
MIT chemical engineers have recently developed model to remove micropollutants from water depend on soap. Soap contains surfactants which have both hydrophobic (water-hating) and hydrophilic (water-loving) components. When water comes in contact with soap, the hydrophobic parts of the surfactant stick together, assembling into spherical structures called micelles with the hydrophobic portions of the molecules in the interior. The hydrophobic micelle cores trap and help carry away oily substances like dirt.
Engineers synthesized micelle-laden hydrogel particles to essentially cleanse water. They used microfluidics which "involve processing fluids on very small, micron-like scales" to generate uniform polymeric hydrogel particles continuously and reproducibly.
These hydrogels, which are porous and absorbent, incorporate a surfactant, a photo-initiator (a molecule that creates reactive species), and a cross-linking agent known as PEGDA. The surfactant assembles into micelles that are chemically bonded to the hydrogel using ultraviolet light. When water flows through this micro-particle system, micropollutants latch onto the micelles and separate from the water. The physical interaction used in the system is strong enough to pull micropollutants from water, but weak enough that the hydrogel particles can be separated from the micropollutants, restabilized, and reused.
Lab testing shows that both the speed and extent of pollutant removal increase when the amount of surfactant incorporated into the hydrogels is increased.
Regeneration of the particles occurs by soaking the micelles in 90% ethanol, whereby "all the pollutants just come out of the particles and back into the ethanol. Ethanol is biosafe at low concentrations, inexpensive, and combustible, allowing for safe and economically feasible disposal. The recycling of the hydrogel particles makes this technology sustainable, which is a large advantage over activated Carbon. The hydrogels can also be tuned to any hydrophobic micropollutant, making this system a novel, flexible approach to water purification.
 English
English Arabic
Arabic


