The coldest chemical reaction in the known universe
In temperatures millions of times colder than interstellar space, Kang-Kuen Ni achieved a feat of precision. Forcing two ultra-cold molecules to meet and react, she broke and formed the coldest bonds in the history of molecular couplings.
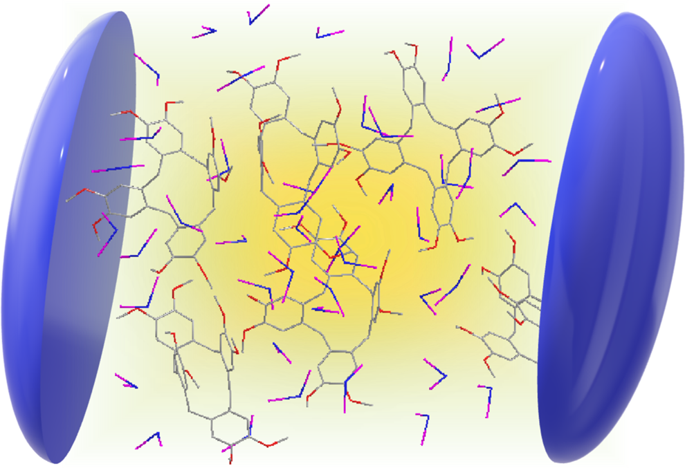
They discovered their new apparatus can do something even they did not predict. In such intense cold -- 500 nanokelvin or just a few millionths of a degree above absolute zero -- their molecules slowed to such glacial speeds, the team could see something no one has been able to see before: the moment when two molecules meet to form two new molecules. In essence, they captured a chemical reaction in its most critical and elusive act.
Chemical reactions are responsible for literally everything: breathing, cooking, digesting, creating energy, pharmaceuticals, and household products like soap. So, understanding how they work at a fundamental level could help researchers design combinations the world has never seen. With an almost infinite number of new combinations possible, these new molecules could have endless applications from more efficient energy production to new materials like mold-proof walls and even better building blocks for quantum computers.
Chemical reactions occur in just millionths of a billionth of a second, better known in the scientific world as femtoseconds. There was no direct measurement of what actually happened in these chemical reactions until now.
Ultra-cold temperatures force reactions to a comparatively numbed speed. Because [the molecules] are so cold. When the team reacted two potassium rubidium molecules -- chosen for their pliability -- the ultra-cold temperatures forced the molecules to linger in the intermediate stage for microseconds. Microseconds -- mere millionths of a second -- may seem short, but that's millions of times longer than usual and long enough for the team to investigate the phase when bonds break and form, in essence, how one molecule turns into another.
Already, the team is exploring what else they can learn in their ultra-cold test bed. Next, for example, they could manipulate the reactants, exciting them before they react to see how their heightened energy impacts the outcome. Or, they could even influence the reaction as it occurs, nudging one molecule or the other.
 English
English Arabic
Arabic