Green method could enable hospitals to produce Hydrogen peroxide in house
A team of researchers has developed a portable, more environmentally friendly method to produce Hydrogen peroxide. It could enable hospitals to make their own supply of the disinfectant on demand and at lower cost.
The main problem is that Hydrogen peroxide is not stable; it starts breaking down into water and Oxygen even before the bottle has been opened. It breaks down even more rapidly once it is exposed to air or light. And because it decomposes so quickly, shipping and storing it become very expensive.
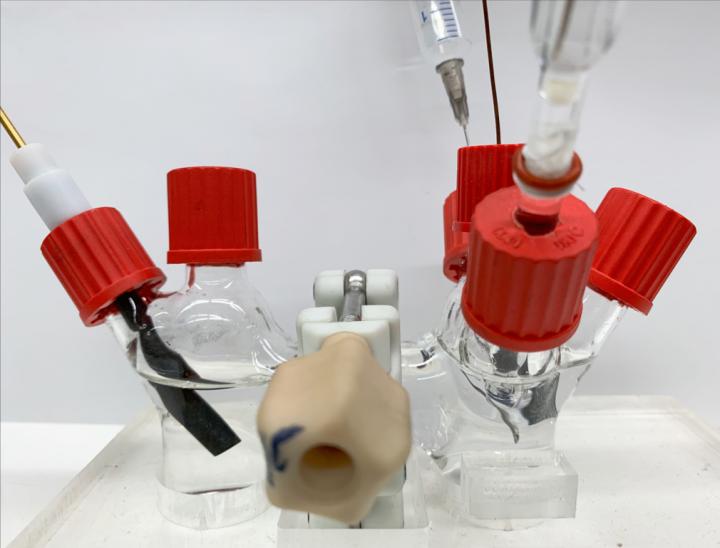
Researchers developed a quick, simple and inexpensive method to generate Hydrogen peroxide in house using just a small flask, air, an off-the-shelf electrolyte, a catalyst and electricity.
Researchers goal is to create a portable setup that can be simply plugged in so that hospitals, and even households, have a way to generate Hydrogen peroxide on demand. No need to ship it, no need to store it, and no rush to use it all before it expires. This could save up to 50 to 70% in costs. Another advantage is that the method is less toxic than industrial processes.
The method is based on a chemical reaction in which one molecule of Oxygen combines with two electrons and two protons in an acidic electrolyte solution to produce Hydrogen peroxide. This type of reaction is known as the two-electron Oxygen reduction reaction, and it is user-friendly because it can produce dilute Hydrogen peroxide with the desired concentration on demand.
The key to making this reaction happen is a special catalyst that the team developed. It is made up of Carbon nanotubes that have been partially oxidized, meaning Oxygen atoms have been attached to the surface. The Oxygen atoms are bound to tiny clusters of three to four Palladium atoms. These bonds between the Palladium clusters and Oxygen atoms are what enable the reaction to occur with a high selectivity and activity due to its optimal binding energy of the key intermediate during the reaction.
 English
English Arabic
Arabic