New ultrahard diamond glass synthesized
Research team synthesized a new ultrahard form of Carbon glass with a wealth of potential practical applications for devices and electronics. It is the hardest known glass with the highest thermal conductivity among all glass materials.
Carbon is unrivalled in its ability to form stable structures alone and in combination with other elements. Some forms of Carbon are highly organized, with repeating crystalline lattices. Others are more disordered, a quality termed amorphous.
The type of bond holding a Carbon-based material together determine its hardness. For example, soft graphite has two-dimensional bonds and hard diamond has three-dimensional bonds.
Because of its extremely high melting point, it's impossible to use diamond as the starting point to synthesize diamond-like glass.
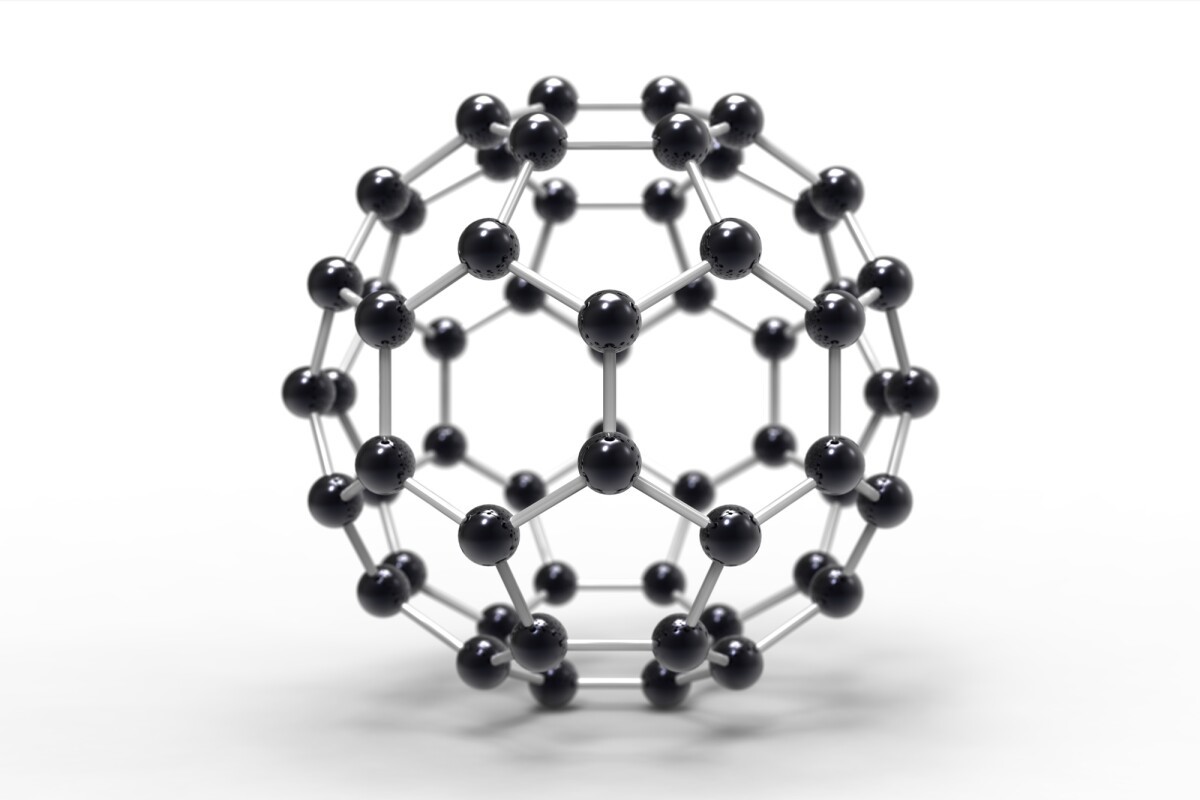
However, the research team, made their breakthrough by using a form of Carbon composed of 60 molecules arranged to form a hollow ball. Informally called a buckyball, this Nobel Prize-winning material was heated just enough to collapse its soccer-ball-like structure to induce disorder before turning the Carbon to crystalline diamond under pressure.
 English
English Arabic
Arabic