A new method for the production of protonated hydrogen
The protonated form of Hydrogen (H+3) has been detected among the molecules that are so sparsely distributed in the vast reaches of the cosmos.
The conditions in outer space can only be described as extreme. The temperature is exceedingly low and radiation is unremitting. It's most certainly a forbidding environment for organisms like us.
Up until now, protonated Hydrogen has only been synthesized on Earth from preformed organic compounds or in highly energized Hydrogen plasmas.
A research group has discovered a new method for the production of protonated Hydrogen (H+3).
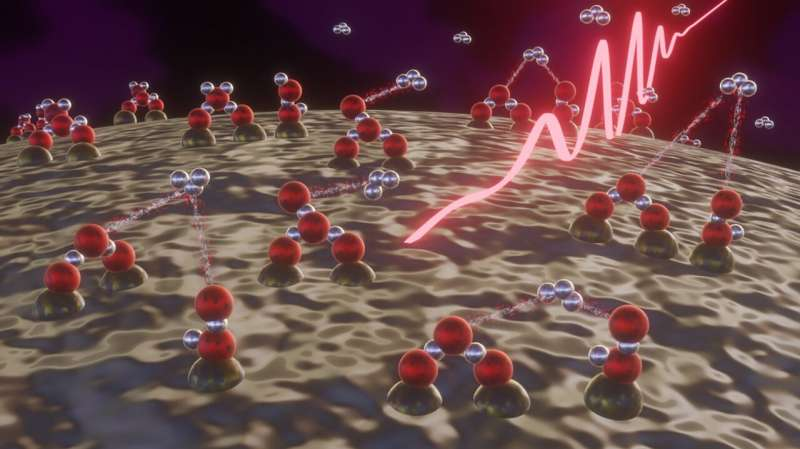
In the experiments, water molecules adsorbed on the surface of Silicon dioxide nanoparticles were irradiated with extremely powerful, ultrashort femtosecond laser pulses, essentially mimicking the effect of the high-energy radiation to which dust/ice particles are exposed in outer space.
The laser light resulted in the ionization and subsequent splitting of the water molecules on the nanoparticles. This sequence of events in turn enabled the tri-Hydrogen cation to be produced via a reaction between pairs of water molecules.
This ionized molecule, H+3 is made up of three protons and two electrons, and takes the form of an equilateral triangle.
The experiments demonstrate that the production of H+3 on ice-coated dust particles can take place in the absence of any other factors. This finding will help to understand how the formation of complex molecules
is driven under the conditions found in outer space.
Owing to its highly reactive nature, protonated hydrogen promotes the formation of more complex hydrocarbons. It is therefore regarded as an important catalyst for the synthesis of organic, Carbon-based molecules.
 English
English Arabic
Arabic