A Quantum Property That Makes Water Weird
Researchers took a new approach to imaging the positions of particles making up liquid water, and to reveal how Hydrogen and Oxygen jostle within water molecules.
They go a long way in fleshing out the quantum modelling of hydrogen bonds, potentially improving theories explaining why water – so vital for life as we know it – has such intriguing properties.
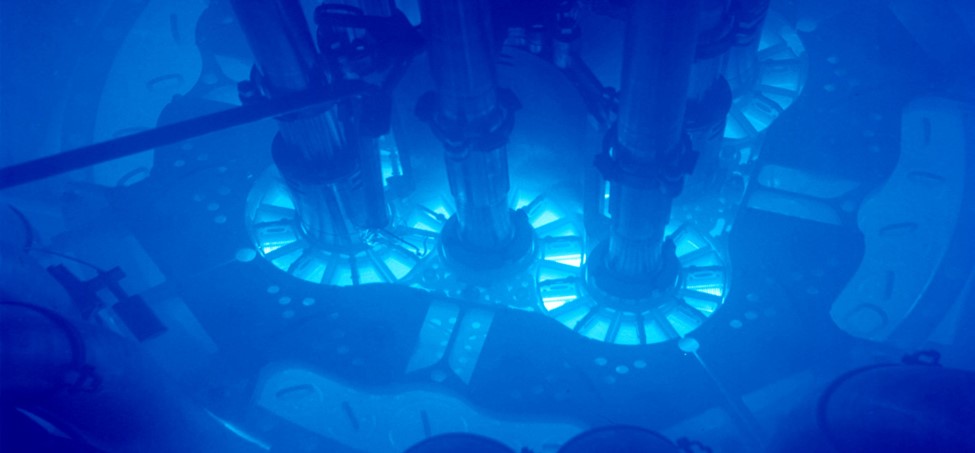
To gain insights into the atoms' arrangements, the team used a Mega-electron-volt Ultrafast Electron Diffraction Instrument. This device showers the water with electrons, which carry crucial information on the atoms' arrangements as they ricochet from the molecules.
With enough snapshots, it was possible to build a high-resolution picture of the jiggle of Hydrogen as the molecules bend and flex around them, revealing how they drag Oxygen from neighbouring molecules towards them before violently shoving them back again.
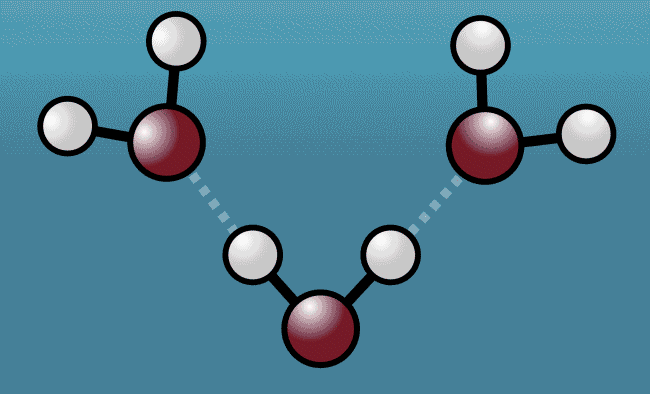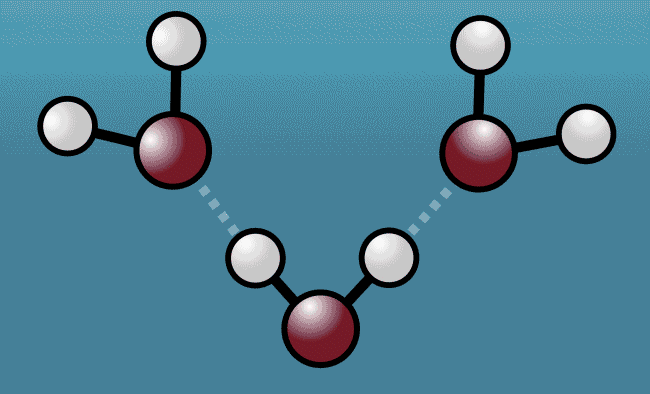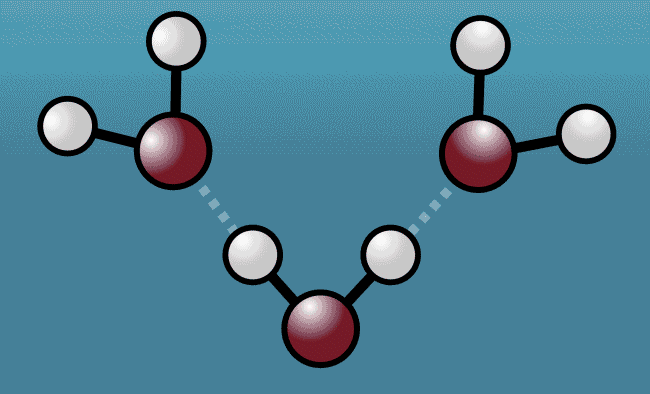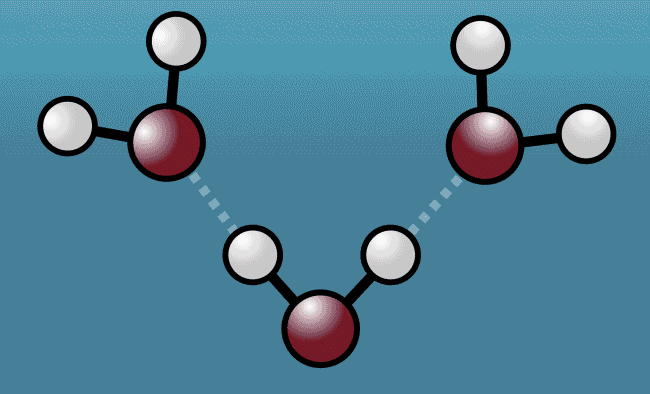
With far more protons than its pair of weenie sidekicks, Oxygen gets slightly more of the molecule's electron love. This leaves each Hydrogen with a little more electron-free time than usual. The tiny atoms aren't exactly left positively charged, but it does make for a V-shaped molecule with a gentle slope of subtly positive tips and a slightly negative core.
Above: Animation shows how a water molecule responds after being hit with laser light. As the excited water molecule starts to vibrate, its Hydrogen atoms (white) tug Oxygen atoms (red) from neighbouring water molecules closer, before pushing them away, expanding the space between the molecules.
This study is the first to directly demonstrate that the response of the Hydrogen bond network to an impulse of energy depends critically on the quantum mechanical nature of how the Hydrogen atoms are spaced out, which has long been suggested to be responsible for the unique attributes of water and its Hydrogen bond network.
Now that the tool has been shown to work in principle, researchers can use it to study the turbulent waltz of water molecules as pressures rise and temperatures fall, watching how it responds to organic solutes or forms amazing new phases under exotic conditions.
 English
English Arabic
Arabic